A Look Back: IPC’s Top 5 Blog Posts of 2019
Comments Off on A Look Back: IPC’s Top 5 Blog Posts of 20192020 marks the start of a new year and a new decade. The start of a new year also provides an opportunity to reflect on what was learned over the past year. At IPC, we’re thrilled to see how the popularity of our blog has grown exponentially over the past year. Some of you liked the blog posts so much, you came back more than once! Here is a review of our most popular posts of 2019.

IPC’s 5 Most Popular Posts of 2019
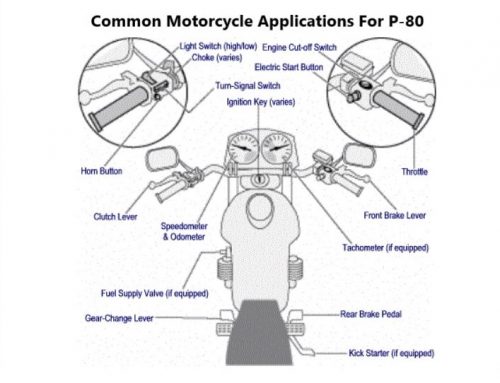
1. 5 Reasons Why P-80 Is the Best O-Ring Lubricant
Remember the last time you had to install an O-ring? Maybe you were assembling new equipment or repairing a pump. Regardless of the type of equipment involved, unless you used an O-ring lubricant chances are you may have struggled with the installation….
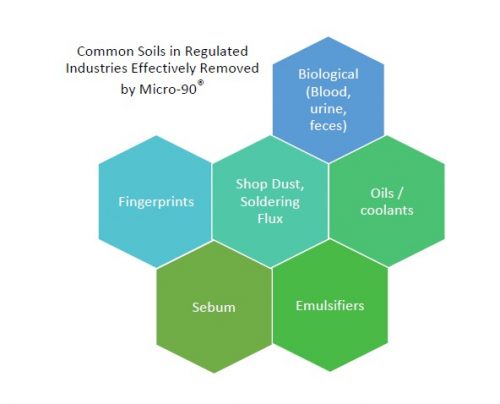
2. Why Micro-90® is A Lab Tech’s Best Friend
Regular cleaning is one of the easiest ways to keep your equipment functioning properly. Apparatus that is not thoroughly cleaned can yield inaccurate and inconsistent results. Trace ingredients from previous use must be removed to avoid cross-contamination and to ensure that all future test results are error-free….
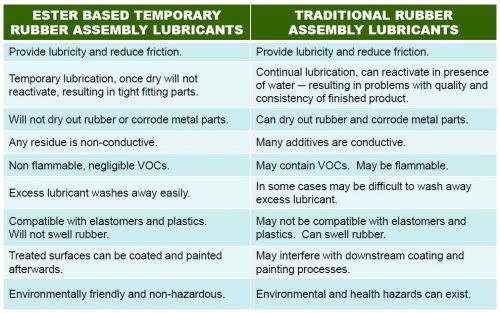
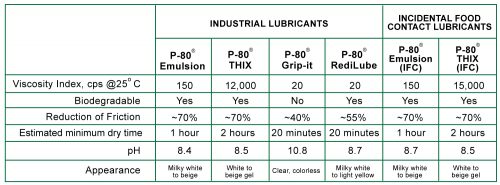
3. Do You Need P-80® Temporary Rubber Assembly Lubricants?
How many times have you pushed a rubber hose onto a fitting only to find it wouldn’t slide into place because the fit was just too tight? Or, how about all of those seals and O-rings that twisted or tore because it took so much force to seat them? Remember the frustration you felt after pushing a grommet into place and then watching it pop right back out?…
4. 5 Step Guide to Choosing The Right Assembly Lubricant
Rubber can be difficult to install, remove or manipulate. It’s not unusual for rubber parts to slip or break during assembly or not fit into place: an O-ring may get twisted, a heater hose may not fully insert, or a gap can appear in a waterproof seal. Improper assembly can lead to a multitude of problems including destroyed parts, invalidated warranty claims, product recalls, and worker fatigue or injury…
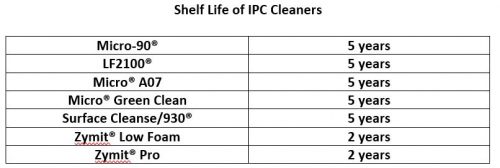
5. Selecting the Right Ultrasonic Cleaning Detergent for Regulated Industries
Regulated industries use ultrasonic cleaning for pharmaceutical equipment, medical devices, surgical equipment, labware, optical instruments, and dental equipment because it is highly effective at removing soil and debris before sterilization, especially on intricate or hard-to-reach parts. A detergent must be used in conjunction with ultrasonic cleaning in order to effectively remove most soils. A multipurpose cleaner is ideal because of its versatility…


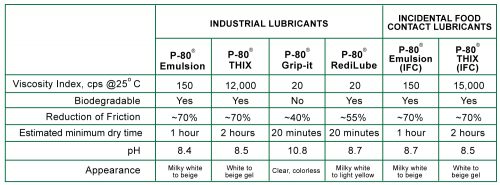


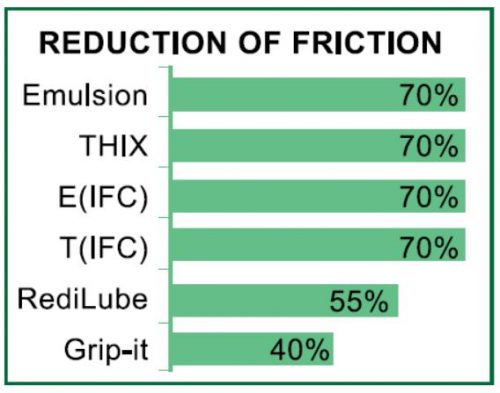
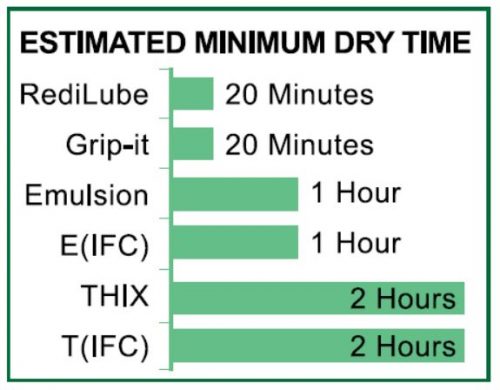


 Common O-ring installation problems include tearing, twisting, turning, pinching and overstretching. Incorrectly installed O-rings may cause leaks which can damage parts and equipment. O-rings that are properly installed can prevent leaks and increase part life.
Common O-ring installation problems include tearing, twisting, turning, pinching and overstretching. Incorrectly installed O-rings may cause leaks which can damage parts and equipment. O-rings that are properly installed can prevent leaks and increase part life.