Why Food Manufacturers Trust IPC’s Cleaners
Disinfectants and other sanitizing agents cannot adequately sanitize a surface with leftover food residue on it. These contaminants can harbor harmful germs and bacteria. Cleaning removes traces of dirt, debris, and dust and primes surfaces and equipment for disinfection. Simply put, cleaning is just as important as disinfecting, and that’s why selecting an appropriate cleaner… Continue Reading Why Food Manufacturers Trust IPC’s Cleaners
Micro Green Clean: A Greener Cleaner Choice
Micro Green Clean: A Greener Cleaner Choice Conventional non-green consumer and commercial cleaning products are potentially hazardous, carrying the risks of long-term illness due to over-exposure, asthma, chemical burns, irritation, or accidental ingestion by a person or pet. As the demand for effective cleaning products grows, manufacturers seek to develop effective formulations that contain more… Continue Reading Micro Green Clean: A Greener Cleaner Choice
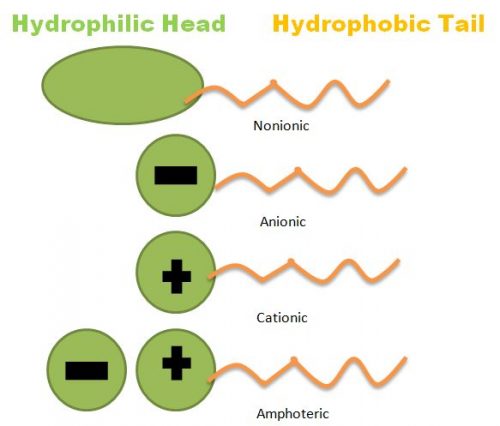
An Easy Guide to Understanding How Surfactants Work
What is a Surfactant? Surfactants are a primary component of cleaning detergents. The word surfactant means surface active agent. As the name implies, surfactants stir up activity on the surface you are cleaning to help trap dirt and remove it from the surface. Surfactants have a hydrophobic (water-hating) tail and a hydrophilic (water-loving) head. The… Continue Reading An Easy Guide to Understanding How Surfactants Work
How to Choose the BEST Cleaner
Choosing the best cleaning product can be confusing. Do you need a neutral product? Alkaline? Enzyme? Sometimes, it’s hard to know. Don’t worry, we’re here to help. We’ve just released the new video “How to Choose the Best Cleaner,” to help you make the best choice. Please click or call us if you have any further… Continue Reading How to Choose the BEST Cleaner
Wastewater Management: What You Need to Know to Clean Filter Membranes
We all know that keeping filter membranes clean is essential to maintaining effluent regulatory compliance and the proper flow of water in a water treatment facility and other industries. A dirty filter means trouble. But cleaning filter membranes can be challenging. That’s where International Products Corporation’s (IPC’s) superior filter cleaners come in. Often copied but… Continue Reading Wastewater Management: What You Need to Know to Clean Filter Membranes
The Importance of Cleaning Before Disinfecting
Disinfecting surfaces to kill traces of microbes and disease is a critical concern right now. A common misconception is that simply disinfecting a surface is enough to sanitize it. This is not the case, cleaning and disinfecting are both important parts of a thorough sanitizing process. Why do both? Surfaces must be properly cleaned prior… Continue Reading The Importance of Cleaning Before Disinfecting

A Look Back: IPC’s Top 5 Blog Posts of 2019
2020 marks the start of a new year and a new decade. The start of a new year also provides an opportunity to reflect on what was learned over the past year. At IPC, we’re thrilled to see how the popularity of our blog has grown exponentially over the past year. Some of you liked… Continue Reading A Look Back: IPC’s Top 5 Blog Posts of 2019

Selecting the Right Ultrasonic Cleaning Detergent for Regulated Industries
Regulated industries use ultrasonic cleaning for pharmaceutical equipment, medical devices, surgical equipment, labware, optical instruments, and dental equipment because it is highly effective at removing soil and debris before sterilization, especially on intricate or hard-to-reach parts. A detergent must be used in conjunction with ultrasonic cleaning in order to effectively remove most soils. A multipurpose… Continue Reading Selecting the Right Ultrasonic Cleaning Detergent for Regulated Industries

What Is The Shelf Life Of My Cleaner? (And, Why It Matters)
Remember that bottle of cleaner that’s been in your cabinet for years? How do you know if it’s still effective and safe to use? These things are good forever, right? Absolutely not! Chemical products do indeed have a shelf life. Paying attention to expiration dates helps ensure you are using products at their peak performance… Continue Reading What Is The Shelf Life Of My Cleaner? (And, Why It Matters)

Why Micro-90® is A Lab Tech’s Best Friend
What Is One Of A Lab Tech’s Biggest Challenges? Cleaning lab equipment, of course! Everyone knows that labware must be properly cleaned, but what’s the best way to achieve that goal? And, why is cleaning so important? Why Clean Lab Equipment? Regular cleaning is one of the easiest ways to keep your equipment functioning properly.… Continue Reading Why Micro-90® is A Lab Tech’s Best Friend
